Beef and Pork Tapeworms Have the Presence of What in Their Stool
General Concepts
Clinical Manifestations
Adult Tapeworms: Adult worms are found in the small intestine; these infections are usually well tolerated or asymptomatic, but may cause abdominal distress, dyspepsia, anorexia (or increased appetite), nausea, localized pain, and diarrhea.
Larval Tapeworms: Larvae locate in extraintestinal tissues and produce systemic infections with clinical effects related to the size, number, and location of cysts. Taenia solium cysticercosis (infection with the cysticercus larval stage) is often asymptomatic and chronic; neurocysticercosis, ophthalmic cysticercosis, and subcutaneous and muscular cysticercosis are, however, frequently reported. Echinococcus granulosus hydatid larvae may form massive cysts in liver, lungs, and other organs, including long bones and the central nervous system.
Structure
Adults, which mature sexually in the definitive or final host, are ribbon-shaped, multisegmented, hermaphroditic flatworms; each segment has a complete male and female reproductive system. An anterior holdfast organ (the scolex) is followed by a germinative portion ("neck") and segments at successively later stages of development. Larvae encyst in various tissues of the intermediate host; larval cysts contain one or many scoleces of future adult worms.
Multiplication and Life Cycle
The tapeworm's life cycle involves a definitive and one or more intermediate hosts (except for the one-host cycle of Hymenolepis nana). Each type of cycle has specialized larval forms (cysticercus, cysticercoid; coenurus, hydatid; coracidium, procercoid, plerocercoid).
Pathogenesis
Pathology due to adult worms results from the physical presence and activity of the large worms (Taenia species), occasional erosive action (causing local inflammation) by scolex hooks (T solium, H nana), or reduced host intake of vitamin B12 (Diphyllobothrium latum). Allergic reactions may also be responsible for symptoms such as headache, dizziness, inanition, and anal and nasal pruritus.
Host Defenses
Adult worms are probably only weakly immunogenic, although some evidence exists for a cell-mediated host response; moderate eosinophilia and increased IgE may occur. Hymenolepis nana, in contrast, elicits a strong immune response when directly infected by the eggs, since the larvae develop within the villi (see page 1105). Larvae elicit strong immunity against reinfection that is derived from both cell-mediated and humoral responses induced by antigenic stimulation of tissues.
Epidemiology
Infective larvae are acquired by eating contaminated raw or undercooked meat, grains, or fish. Taenia solium cysticercosis or H nana can be transmitted in a direct cycle via ingestion of eggs from human feces. Echinococcus eggs from dog or fox fur cause human hydatid disease (humans are the intermediate host; canids are the definitive hosts). Reinfection with adult tapeworms is common; second infections with larvae are rare. T solium cysticercosis may be acquired by autoinfection; internal autoinfection with H nana from a cysticercoid infection is possible.
Diagnosis
Adult Worms:Taenia infections are diagnosed by finding gravid segments in stool specimens; the eggs of these species are indistinguishable. Other species are diagnosed on the basis of eggs in stool specimens.
Larval Worms: Cysts in tissues may be identified in biopsy specimens, by radiography (calcified cysts), and by computed tomography (brain cysts). Serology (indirect hemagglutination, ELISA) is useful but of variable sensitivity and specificity. A history of travel in endemic areas is often of great importance.
Control
Meat should be cooked thoroughly or frozen at -10°C for 10 days; beef and pork should be inspected for Taenia ("measly meat"); human feces should not contaminate drinking water; sheepdogs should be treated and should not be fed sheep viscera. Humans may be treated with praziquantel or niclosamide.
Introduction
Tapeworms are ribbon-shaped multisegmented flatworms that dwell as adults entirely in the human small intestine. The larval forms lodge in skin, liver, muscles, the central nervous system, or any of various other organs. Their life cycles involve a specialized pattern of survival and transfer to specific intermediate hosts, by which they are transferred to another human host. Each pattern is characteristic of a given tapeworm species.
In general, the common gut-dwelling adult cestodes are well adapted to the human host, induce few symptoms, and only rarely cause serious pathology. This reality belies innumerable fearsome and largely apocryphal stories of tapeworms stealing food and causing ravenous hunger (far more commonly, the appetite is depressed). Larval cestodes, however, develop in human organs or somatic tissues outside of the gut and are therefore far more pathogenic.
The adult cestodes elicit little host inflammatory or immune response in contrast to the strong responses elicited by the larval stages in tissues. Adult cestodes are often acquired by ingestion of meat from intermediate hosts. Extraintestinal infection with larvae results from ingestion of eggs of fecal origin. Diagnosis of infection with adult cestodes is based on identification of eggs and segments (proglottides) in feces. Larval infections are more difficult to assess; serology and biopsy are helpful. Control depends on sanitation, personal hygiene, and thorough cooking of meat and fish.
Taenia saginata, The Beef Tapeworm
Clinical Manifestations
The clinical manifestations of infection with adult T saginata tapeworms are confined to occasional nausea or vomiting, appetite loss, epigastric or umbilical pain, and weight loss. Moderate eosinophilia may develop. A disturbing manifestation of T saginata infection is the active crawling of the muscular segments out of the anus. Rarely, intestinal perforation may occur from the scolex of Taenia, or proglottides may be vomited and then aspirated.
Structure
Adults are ribbonlike, flattened, segmented, hermaphroditic flatworms 5 to 10 m long, consisting of scolex, neck, and immature, mature, and ripe segments in linear sequence. The distinctive morphologic and physiologic properties of the adult tapeworm reflect on the one hand their remarkable specialization for survival in the vertebrate intestine, and on the other hand their massive reproductive powers which are made possible by the multiple sexual units, the proglottides or segments. This ensures the worm species against the enormous rate of loss of the segments or eggs passed in the feces, with only the most remote probability of any one egg succeeding in reaching an intermediate host and being transferred to another human. The terminal one-third to one-half of the worm's length consists of gravid (egg-filled) segments. These segments are muscular and can crawl caterpillar-fashion through the anal sphincter to the outside environment—which renders them available to their herbivore intermediate hosts.
The larval cyst of T saginata—the cysticercus—is a pea-sized, fluid-filled cyst, which develops in the muscles of the intermediate host. Within the cyst is a single inverted scolex, formed from a germinative portion of the inner cyst wall (Fig. 89-1).

Figure 89-1
Larval types found in the taeniid tapeworms. . (From Muller R: Worms and Disease: A manual of Medical Helminthology. William Heinemann Medical Books, London, 1975, with permission.)
Multiplication and Life Cycle
Figure 89-2 illustrates the life cycle of T saginata. Gravid segments break off from the worm and are carried in the fecal bolus or by their own crawling activity to the soil. The segments move away from the bolus and adhere to grass. If ingested by a bovine intermediate host, the segments are digested open in the gut, each releasing 50,000 to 100,000 eggs. The eggs hatch, each releasing a six-hooked larva, the oncosphere (also called the hexacanth), which penetrates the gut wall and reaches the muscles via the circulation. There the oncosphere fills with fluid and develops into the 8-mm cysticercus. If a human eats raw or undercooked infected beef, the cysticercus is digested free and inverts the scolex, which attaches to the wall of the small intestine and begins to bud off the long chain of segments. In about 3 months the worm reaches 4-5 m in length and gravid segments begin to pass through the anus. The worm is long-lived, surviving 5 to 20 years or more.
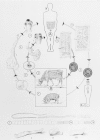
Figure 89-2
Life cycles of Taenia solium and T saginata. . (a) The port (pig) tapeworm (outer developmental cycle); (b) the beef (cattle) tapeworm (inner developmental cycle). A. Final host: humans only. Tapeworm in the small intestine
Pathogenesis
Rare intestinal blockage or penetration have been reported, but pathology is usually inconsequential—although the psychological distress at passing motile segments may be extreme.
Host Defenses
Because of its limited contact with the epithelial lining, the gut-dwelling adult tapeworm induces little host inflammatory, allergic, cell-mediated, or humoral response. The sucking action of the scolex appears to have relatively limited immunogenic effect. The long life span of the worm suggests the absence of an effective inhibitory mechanism.
Epidemiology
Taenia saginata, the commonest large tapeworm of humans, is transmitted as cysticerci in beef ("measly beef"). Partially cooked, smoked, or pickled beef can be infective, although raw beef (steak tartare) is the commonest bearer of infection, as witnessed by the frequency of taeniasis in countries such as Ethiopia and Argentina where raw or undercooked beef is often eaten.
Large worms may grow by 15 to 30 cm a day in the human gut, passing 10 segments daily, which may convey up to a million eggs a day into the environment throughout the long life span of the worm. Eggs may also be found in pastures flooded by human sewage or on which human sewage is used as fertilizer.
Diagnosis
Adult infections can be diagnosed by identifying segments in the feces. The species of Taenia can be identified only by the segments, because their eggs are identical. The uterus of Tsaginata usually forms 12 to 20 branches on each side of the main uterine stem, whereas there are 7 to 10 branches in the smaller and relatively wider T solium segment (see Fig. 89-2).
Control
Inspection of beef for cysticerci is the best preventive measure. Beef must be thoroughly cooked in endemic areas—to at least 56°C throughout the meat, which may be difficult to accomplish with large cuts of fatty meat, particularly pork. Freezing at 10°C for 10 days usually is lethal to Taenia cysticerci, but they can withstand 70 days at 0°C.
Treatment is readily available for the intestinal adult worms. Niclosamide, is a nonabsorbed oxidative phosphorylation inhibitor that kills the scolex and anterior segments on contact, after which the worm is expelled. Praziquantel, a synthetic isoquinoline-pyrazine derivative, is an equally effective and relatively nontoxic cesticidal compound. Since the scolex is usually but not always destroyed, and a new worm can regenerate if the scolex and a minute portion of the neck survive, the patient should be observed for several months, as egg-bearing segments can reappear in 10-12 weeks.
Taenia solium, The Pork Tapeworm
Clinical Manifestations
The clinical effects of adult T solium infection are similar to those caused by T saginata.
Structure
The scolex of T solium differs from that of T saginata in possessing an anterior circle of sharply spined hooks arranged in a double row. These are under muscular control and work with the four suckers to adhere to the gut wall. As described above, gravid segments of Tsolium can be distinguished from those of T saginata by the number of outpocketing branches of the uterus. The adult worm is usually 3 to 5 m long. Gravid segments tend to be less muscular and more square than those of T saginata.
Multiplication and Life Cycle
As shown in Figure 89-2, the life cycle of T solium is similar to that of T saginata except that the pig is the principal intermediate host. Because the gravid segments are less motile than those of T saginata, they are usually eliminated in the fecal matter and remain in the fecal bolus (which increases the chance of infecting pigs, which are coprophagous).
Of great clinical importance is the fact that humans who ingest eggs from human feces, as distinct from cysticerci in undercooked pork, may develop the larval infection just as pigs do, resulting in the serious disease cysticercosis.
Pathogenesis
The hooked scolex of T solium may cause greater intestinal disturbance, pain, and inflammatory response than that caused by T saginata, but symptoms are still generally mild and the pathology minor. However, T solium larval infection (cysticercosis) is a potentially dangerous systemic infection, the degree of damage depending on the site and number of cysticerci that develop. Infection most commonly occurs in the central nervous system (CNS). It is also frequently found in muscles and subcutaneous tissues. The globe of the eye is also a common site. In the CNS, the larvae most often occupy the brain hemispheres. They may also be found in the cisternae and ventricles. Hydrocephalus may result from obstruction of cerebrospinal fluid flow. Infection in specific sites can induce epilepsy, mental disturbances, or a meningeal syndrome. Nevertheless, up to one-half of CNS infections are thought to be asymptomatic. After death of the scolex within the cyst—often years after infection—the capsule becomes fibrosed or calcified.
Host Defenses
Owing to the systemic migration and tissue localization of the cysticerci, cysticercosis elicits considerable host sensitization. This response is usually insufficient to block the initial infection but probably renders the normal host immune to a subsequent one. Much of the damage from cysticercosis is caused by the severe inflammatory host response that occurs after the death and disruption of the parasite .
Epidemiology
A remarkable and tragic aspect of T solium infection is the ability of this worm to develop both adult and larval stages in humans. If T solium eggs are ingested (from fecally contaminated water or by anus-to-mouth transfer of infective eggs), they may hatch in the gut and spread systemically, causing human cysticercosis. It appears likely (although it is unproven) that human cysticercosis may also be caused when reverse peristalsis, induced by adult T solium in the gut, returns gravid segments into the duodenum, where the eggs hatch and release invasive oncospheres. Cysticerci develop to potency in about 3 months and may live many years. Cysticerci that die may become calcified, rendering them demonstrable by radiography.
Human cysticercosis is a serious and widespread disease, being especially common in Latin America. The disease is frequently found among Mexican agricultural workers in California and other Western states. Human cysticercosis apparently cannot develop from eggs of T saginata.
Diagnosis
Diagnosis of adult worm infection is similar to that for T saginata. Cysticercosis is difficult to diagnose and usually requires radiologic, serologic, and clinical assessment. Subcutaneous nodules can usually be felt or observed, and can be sampled by biopsy. The enzyme-linked immunosorbent assay (ELISA) is useful, especially with the purified antigens that are now available. Plain radiographs of soft tissues may demonstrate the oval or elongated cysts (4-10 mm × 2-5 mm) if they are wholly or partially calcified. Cysts in muscles are usually aligned with the fibers. Soft tissue or brain calcifications are strongly indicative of cysticercosis. Plain skull films may show cerebral calcifications or indicate intracranial hypertension. Computed tomography is the most useful procedure, as it detects calcified and noncalcified cysts as well as edema or intracranial hypertension.
Control
The control of infection of humans as definitive hosts is the same as that for T saginata, except that the control measures apply to pork not beef. In addition, human sewage from infected individuals may contaminate the source of drinking water. The eggs are highly resistant and can withstand many months of environmental exposure over a broad temperature range. Treatment for adult T solium is the same as for T saginata.
Cysticercosis may require surgery for ophthalmic or brain involvement, but chemotherapy should precede surgery when possible. Tissue infection can be treated with albendazol (taken with a fatty meal to increase absorption or praziquantel (combined with corticosteroids to reduce the inflammatory response to the dead cysticerci). Praziquantel should not be used for ocular or spinal cord infections.
Taenia multiceps, The Coenurus Tapeworm
The adult worm of Taenia multiceps is found in dogs or wild canids. The larva is a bladderworm with multiple scoleces—from a few to 100 or more—in an encysted vesicle. This vesicle, usually 2 - 5 cm in diameter, is called a coenurus (Fig. 89-1). The usual intermediate host is the sheep. Human infection can occur from accidental ingestion of dog feces containing the eggs.
Infection in humans usually occurs in the brain in temperate areas, and in the eye or subcutaneous tissues in tropical areas. Diagnosis and treatment are similar to those for Echinococcus infection, which may be difficult to distinguish from coenurus infection. Treatment is chiefly surgical, although the drugs used for cysticercosis may also be effective against coenurus infection.
Echinococcus granulosus, The Hydatid Tapeworm
Clinical Manifestations
Echinococcosis (hydatid disease) results from the presence of one or more massive cysts, or hydatids, which can develop in any tissue site, including the liver, lungs, heart, brain, kidneys, and long bones. The clinical manifestations of this infection therefore vary greatly, depending on the site and size of the cyst, but resemble those of a slow-growing tumor that causes gradually increasing pressure. Infections in the liver, lungs, or subcutaneous tissue sites may be asymptomatic for many years, but pressure effects eventually develop. In sensitive or vital areas, hydatids produce a panoply of symptoms, chiefly owing to mechanical compression or blocking effects but also include collapse of infected long bones, blindness, and epileptiform seizures. The rupture of a hydatid cyst may induce sudden anaphylactic shock in a previously asymptomatic individual.
Structure
Adult E granulosus tapeworms are relatively minute, consisting of 3 to 5 segments, and usually are less than 1 cm long. Dogs and wild canids are the only final hosts in which the adults are found, often adhering in great numbers to the small intestinal mucosa. The scolex has four suckers and is crowned with a circle of spines as in T solium. It is followed by a germinative neck region, one developing segment, and usually one gravid segment containing several hundred eggs.
The hydatid larva is found in sheep, in many other herbivores—and in humans. In humans the cyst is slow-growing, but in a period of years may reach a diameter of 30 cm with a 1 mm thick, laminated sheath surrounded by fibrous reactive host tissue. The cyst is usually fluid-filled and, if viable, has a germinative inner lining from which many thousands of scoleces are budded off into the lumen or remain attached to the germinative wall (Fig. 89-1). The floating scoleces often enlarge, become vesicular, and develop into daughter floating colonies within the parent cyst. These in turn may bud off a third generation of cysts within themselves. The result is an enclosed sac containing myriads of future adult worms ready to infect a dog or other susceptible carnivore that feeds on the hydatid-infected animal and the scolex-filled cyst fluid.
Multiplication and Life Cycle
Echinococcus granulosus causes a zoonosis; the adult is a parasite of canids. The dog-sheep cycle (Fig. 89-3) is the one most germane to humans Other animal cycles, such as dog-camel, dog-horse, wolf-moose, wolf- or coyote-deer, also occur, each with a degree of specific host-parasite adaptation, as well as a distinct geographic range. Eggs passed by the dog can be ingested by sheep or other herbivores, or by humans who have close contact with feces-contaminated dog fur. Within the sheep or human intermediate host, the eggs hatch, the oncospheres penetrate the gut, migrate, and ultimately one or several may form the enormous hydatid cysts. Because the scoleces in the hydatid fluid resemble sand grains, they are called hydatid sand. If a cyst bursts within the human body, it can give rise to dozens of new cysts—limited, in most cases, by a strong cellular immune response of the host.

Figure 89-3
Life cycles of Echinococcus granulosus and E multiocularis. A. Final host: dog (and other canids) 1.a. Echinococcus granulosus (beside it, the worm is reproduced approximately half its natural size)
Pathogenesis
Jaundice and portal hypertension can result from pressure effects of a cyst in the liver; hemoptysis and dyspnea from a lung cyst; and acute inflammatory effects may follow brain or spinal cord infections.
Host Defenses
The migrating and growing larvae, and antigens that leak from the cyst, induce a strong immune response—but rarely one capable of penetrating and destroying the cyst. Ruptured cysts may cause anaphylaxis and the appearance of new cysts in other sites, suggesting an active but ineffectual immune response.
Epidemiology
Echinococcus granulosus is most common in temperate sheep-raising areas: southern South America, the southern and central Russia, East Africa, and the western United States. The source of most human infections is sheepdog feces containing E granulosus eggs (which often adhere to the fur of dogs petted by humans). Killed sheep fed to dogs maintain the infection; sheep ingest eggs with dog feces in their grazing. Other species of Echinococcus are also found in Africa, South America, and elsewhere, sustained by similar canid-prey relationships.
Diagnosis
Symptoms of a tumorlike, slowly growing mass with eosinophilia are strongly suggestive, especially in an endemic sheep-raising area. Isolated hooks in the sputum suggest rupture of a lung cyst. The serologic tests that are currently most useful are indirect hemagglutination, latex agglutination, and ELISA. However, both false positives and false negatives are frequent and may have dangerous repercussions (see Morris and Richard, 1992; Schantz et al., 1990). Ultrasound imaging (US), magnetic resonance (MR), and computed axial tomography (CT) have greatly improved the diagnosis of deep-seated lesions and also can demonstrate avascular fluid-filled cysts. Radiographs are less precise or useful, although they demonstrate the hollow cyst areas.
Control
Effective control is chiefly epidemiologic: denying sheep dogs access to carcasses of infected sheep, obligatory testing and treatment of all sheep dogs, prevention of contact of children with possibly infected sheep dogs, and widespread education on the danger and method of spread of hydatid disease.
Treatment is chiefly by surgical resection (with extreme care to avoid or decontaminate spillage). Recent work suggests that a long course of albendazole may kill the scoleces within the cyst and even reduce the size of the cyst. Long, continued use of mebendazole has also proved effective, although the results are variable. A recent approach involves percutaneous puncture under sonographic guidance, aspiration of cyst fluid, instillation of a protoscolecidal agent such as 95% methanol or cetrimide, and respiration (PAIR), along with albendazole treatment to reduce the danger of subsequently renewed disease from spillage (see Giorgio et al., 1992).
Echinococcus multilocularis, The Multiloculate or Alveolar Hydatid Tapeworm
Echinococcus multilocularis, which normally follows a fox-rodent cycle in northern Siberia and North America, is occasionally conveyed to human fur trappers via fox pelts. In humans it causes a frequently fatal form of echinococcosis. The appearance and life cycle of this cestode closely resemble those of E granulosus, except for the restricted range and small number of hosts. The cyst, however, is extremely dangerous as it lacks the laminated membrane that confines the cyst of E granulosus, and develops an invasive, uncontrolled series of connected chambers (hence the designation "multiloculate" and the alternative name alveolar hydatid). It therefore resembles a malignant growth, capable of budding off to cause metastatic spread. The primary cyst usually forms in the liver. The disease is usually diagnosed late, when it is inoperable, and ends fatally. Early radiologic imaging by US, CT, or MR is essential. Serological tests, particularly with purified E multilocularis antigens, are sensitive and highly specific. Treatment with mebendazole, albendazole, or praziquantel, and surgery should follow.
Hymenolepis nana, The Dwarf Tapeworm
Clinical Manifestations
Hymenolepis nana infections are often asymptomatic, especially in light cases. Heavy infections can induce enteritis with nausea and vomiting, diarrhea, abdominal pain, and dizziness. Massive infection with several thousand worms may follow autoreinfection.
Structure
These small worms, 15 to 50 mm long, have minute segments that are wider than long, a foursucker scolex with a retractable spined anterior rostellum, and terminal gravid segments that break up and release their egg load after they are caught up in the fecal bolus.
The larval form is a cysticercoid, a tailed structure that has a withdrawn scolex and lacks a fluid filled bladder. Typically, Hymenolepis larvae are found in insect or crustacean intermediate hosts—with the sole and remarkable exception of H nana, whose cysticercoid larvae can develop either in an insect or in the small intestinal villi of its human (or rodent) final host.
Multiplication and Life Cycle
The life cycle of this parasite is shown in Figure 89-4. Infection is acquired most commonly from eggs in the feces of another infected individual, which are transferred in food, by contaminated fingers, or in sewage-contaminated drinking water.
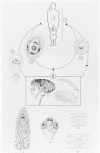
Figure 89-4
Life cycle of Hymenolepis nana. A. Final host: humans (also dog, rodents) 1. Hymenolepis nana, sexually mature worm, about 1/2 natural size
The ingested eggs hatch in the duodenum, and the oncospheres penetrate only into the villi (Fig. 89-5). There, each oncosphere forms a cysticercoid larva that emerges, 4 to 5 days later, into the gut lumen as a young scolex and neck; the scolex attaches to the mucosa, the neck proceeds to strobilate, and the worm reaches full size in 5 to 10 days. The adult worm sheds gravid terminal segments, which disintegrate in the intestine, releasing eggs that are passed in the feces. When these eggs are ingested by another (nonimmune) human, this direct or one-host life cycle begins again. Worms live only a short time, perhaps 4 to 6 weeks. Rodents also can harbor these worms and may serve as reservoir hosts, infecting humans via their pellets.

Figure 89-5
Diagrammatic section of an adult and larval H nana in the gut of a mouse. . In heavy infections in this host, much of the mucosal lining is abraded. (From Muller R: Worms and Disease: A Manual of Medical Helminthology. William Heinemann Medical Books, (more...)
Remarkably, an indirect, two-host cycle may also occur, involving grain beetles, fleas, or other insects that feed on contaminated rodent droppings. Insects that ingest the H nana eggs can serve as hosts for the cysticercoid larvae. Humans who accidentally ingest infected grain beetles (some of which, such as Tribolium, are only 2 to 3 mm long) digest the cysticercoid free; digestive enzymes then act on the cysticercoid to release the scolex, which attaches and develops by this indirect cycle into an adult worm identical to that acquired by the direct life cycle.
A third mode of infection is by internal infection or autoreinfection. Eggs from worms acquired in an initial infection—probably via the indirect, insect cycle, which is nonimmunizing (see Host Defenses, below)—can hatch, invade the villi, and produce a second generation of worms. Since many eggs can be involved, this pathway can lead to massive infection with several thousand worms.
Pathogenesis
Little or no pathology occurs from development of cysticercoids in the villi, and only after a heavy infection (perhaps produced by autoreinfection) do symptoms develop from the adult worms. Children may be particularly subject to massive worm loads and show the most severe intestinal symptoms.
Host Defenses
The tissue phase of the direct cycle of H nana infection (Figs. 89-4 and 89-5) initiates a profound cellular and humoral response, rendering most hosts immune to subsequent infection (as demonstrated experimentally in rodents). In contrast, the indirect cycle through infected insects does not involve mucosal embryogenesis in humans and induces little or no immunity, even permitting occasional massive internal reinfection to occur. The immune response is seldom effective against the initial infection because the tissues have already been invaded and a protective cyst formed by the time the response develops.
Epidemiology
Hymenolepis nana is probably the commonest human cestode, owing to its wide distribution, particularly in crowded areas, such as India and China. The direct infectiousness of the eggs frees the parasite from its former dependence upon an insect intermediate host, making rapid infection and person-to-person spread possible. The short life span and rapid course of development also facilitate the spread and ready availability of this worm. Congested areas, day-care centers, and crowded institutions such as prisons frequently have high levels of infection with H nana, despite its strong immunizing capacity and short life span.
Diagnosis
H nana infections can be diagnosed accurately and rapidly by inspecting the stool for eggs.
Control
Preventing fecal contamination of food and water in institutions and crowded areas is of primary importance. General sanitation and rodent and insect control (especially control of fleas and grain insects) are also essential for prevention of H nana infection. Treatment with praziquantel or niclosamide is usually effective, and can be repeated if necessary.
Hymenolepis diminuta, The Rat Tapeworm
The rat tapeworm, which is larger than H nana (up to 40 cm long), has a life cycle involving grain insects, similar to the indirect cycle of H nana. H diminuta rarely infects humans, but may do so if a human eats an insect carrying cysticercoids of this worm. The infection is most common in children, causes a mild diarrhea, is diagnosed by finding the characteristic eggs in the stool, and is readily treated with praziquantel.
Dipylidium caninum, The Double-Pored Tapeworm
Dipylidium caninum causes a cosmopolitan infection of dogs and cats. Fleas are the intermediate hosts in which the infective cysticercoids develop. Children in close and continuous contact with pets are occasionally infected as a result of the accidental ingestion of an infected flea. The infection is usually asymptomatic and is self-limited, although praziquantel would probably be an effective treatment. Flea control of pets would largely eliminate the infection from household pets and children.
Diphyllobothrium latum, The Broad Fish Tapeworm
Clinical Manifestations
Infection with Diphyllobothrium latum is usually asymptomatic, although occasional diarrhea, abdominal pain, fatigue, vomiting, dizziness, or numbness of fingers and toes may be present. Eosinophilia develops during the early stages of worm growth.
Structure
Diphyllobothrium latum is the largest parasite of humans, reaching lengths up to 10 m and consisting of a chain of 3,000 to 4,000 segments, each up to 2 cm wide. The adult worm, a member of the order Pseudophyllidea, is characterized by a scolex with a pair of linear sucking grooves instead of suckers and hooks, and by having a rosette-shaped uterus connected to the outside by a uterine pore through which the eggs are passed. Hence, mature segments produce eggs until they die and are shed, rather than by breaking off as intact egg-filled segments, as in Taenia. Up to a million eggs can be produced daily. The developmental stages are (1) the ciliated, swimming coracidium that hatches from the egg, (2) the procercoid that develops in the copepod primary host, and (3) the plerocercoid (or sparganum), a nonencysted, nonsegmented larval worm, 20 mm or more in length, found in the fish secondary hosts. The plerocercoid develops into the adult tapeworm in the small intestine of a fish-eating final host, such as human, cat, dog, or bear.
Multiplication and Life Cycle
Diphyllobothrium latum is the only adult cestode of humans that has an aquatic life cycle (Fig. 89-6). Eggs are passed in feces of an infected human (or bear, dog, cat, wolf, raccoon, or other freshwater fish-eating reservoir host). If passed into lake or pond water, the eggs develop in 2 or more weeks (varying with the temperature) and hatch, releasing the spherical ciliated coracidium that contains the oncosphere. When ingested by an appropriate water flea (copepods such as Cyclops or Diaptomus), the coracidium sheds the ciliated coat, penetrates into the hemocoel, and changes in 2 to 3 weeks into the 0.5 mm, tailed second-stage embryo, the procercoid. If the infected copepod is then ingested by a minnow or other fish, the procercoid penetrates the fish gut in a few hours and later develops into a third-stage larva, the plerocercoid or sparganum. Usually, these small infected fish are eaten by larger ones; in each new fish host, the plerocercoid penetrates into the fascia or muscles. Eventually, a large game fish, such as a perch or pike, is infected; after being eaten by a human, the fish releases its tapeworm passenger, which attaches and begins adult life. In a few months, the worm is 5 to 10 m long.

Figure 89-6
Life cycle of Diphyllobothrium latum. A. Final host: Humans, dogs, cats (and other fish-eating domesticated and wild animals). Site of the tapeworm: small intestine 1. Egg after it is passed in feces
Pathogenesis
Infection with this tapeworm usually produces no pathology, although the minor symptoms noted above are occasionally present. Megaloblastic anemia ("tapeworm anemia"), which is exacerbated by the worm's uptake of vitamin B12, is now seldom seen, as a result of improved diet, prenatal care, and ready treatment. This condition was formerly most common in Finland.
Host Defenses
Little or no protective immunity develops, owing to the lack of an intimate tissue phase in the human host. Reinfection is common.
Epidemiology
Infection with the broad fish tapeworm is common in temperate and subarctic regions, wherever freshwater fish are eaten raw—as in Scandinavia, Siberia, the Great Lakes, Japan, central Europe, and Chile.
Diagnosis
The ovoid, operculated eggs passed in abundance in the human stool are diagnostic. Occasionally, strands of exhausted segments with the characteristic rosette-shaped uteri are also passed.
Control
Plerocercoids in fish are quickly killed by thorough cooking, freezing at -10°C for 15 minutes, or thorough pickling. Treatment of sewage before it enters lakes greatly reduces the prevalence of infection, as has been demonstrated in Finland. Treatment with praziquantel or niclosamide is effective and nontoxic.
Spirometra
Sparganosis is a tissue infection with the sparganum (or plerocercoid) of Spirometra, a genus related to Diphyllobothrium. These two genera have similar life cycles, but Spirometra usually utilizes frogs, reptiles, or various small mammals as intermediate hosts, whereas Diphyllobothrium uses fish. In the Southeast Asia, frog flesh (rather than beef steak) is used as a poultice over a wound or black eye, which allows the sparganum to crawl into the wound or orbit, initiating a severe inflammatory response. Humans can also acquire the infection as a result of drinking water containing infected Cyclops and possibly from undercooked snake or other infected meat. The procercoids from Cyclops invade the gut wall of the human or animal intermediate host and usually migrate to subcutaneous tissues to form a sparganum, which induces in humans formation of a fibrous 2-cm nodule that encloses and destroys the worm. The nodule can usually be removed surgically or can be treated with praziquantel if the cyst is inaccessible to surgery.
References
-
Adamson ML, Caira JN. Evolutionary factors influencing the nature of parasite specificity. (Review) Parasitology. 1994;109 Suppl:S85–95. [PubMed: 7854854]
-
Desnos M, Brochet E, Cristofini P. et al. Polyvisceral echinococcosis with cardiac involvement imaged by two-dimensional echocardiography, computed tomography and nuclear magnetic resonance imaging. Am J Cardiol. 1987;59:383. [PubMed: 3812300]
-
Flisser A. Taeniassis and cysticercosis due to Taenia solium. (Review) Progress in Clinical Parasitology. 1994;4:77–116. [PubMed: 7948938]
-
Gemmell MA, Lawson JR, Roberts MG. Control of echinococcosis/hydatidosis. Present state of worldwide progress. Bull WHO. 1986;64:333. [PMC free article: PMC2490883] [PubMed: 3490317]
-
Ito A, Smyth JD: Adult cestodes. In Soulsby EJL (ed): Immune Responses in Parasitic Infections: Immunology, Immunopathology, and Immunoprophylaxis. Vol. 2. Trematodes and Cestodes. CRC Press, Boca Raton, FL, 1987 .
-
Kammerer WS, Schantz PM: Echinococcal disease. In Maguire JH, Keystone JS (ed): Infectious Disease clinics of North America. Parasitic Diseases 7(3):467, Saunders, Philadelphia, 1993 .
-
McCormick GF. cysticercosis—Review of 230 patients. Bull Clin Neurosci. 1985;50:76. [PubMed: 3842090]
-
Morris DL, Richards KS: Hydatid Disease: Current Medical and Surgical Management. Oxford, Butterworth-Heinemann LTD., 1992 .
-
Pawlowski ZS: Cestodiases: taeniasis, cysticercosis, diphyllobothriasis, hymenolepiasis, and others. In Warren KS, Mahmoud AAF (eds): Tropical and Geographical Medicine. 2nd Ed. McGraw-Hill Information Services, New York, 1990 .
-
Schantz PM: Cestode diseases. In Goldsmith R, Heyneman D (ed): Tropical Medicine and Parasitology. Appleton & Lange, East Norwalk, CT, 1989 .
-
Sortelo J, Escobedo F, Penagas P. Albendazole vs praziquantel for therapy of neurocysticercosis. Arch Neurol. 1988;45:532. [PubMed: 3358706]
-
St. Geme JW, Maldonado YA, Enzmann D. et al. Consensus: diagnosis and management of neurocysticercosis in children. Pediatr Infect Dis J. 1993;12:455. [PubMed: 8345976]
earlsoripsensfuld.blogspot.com
Source: https://www.ncbi.nlm.nih.gov/books/NBK8399/
0 Response to "Beef and Pork Tapeworms Have the Presence of What in Their Stool"
Post a Comment